Bedfont® Scientific Limited hosted an informative webinar Children with Asthma, with seasoned respiratory nurse Carol Stonham MBE leading the discussion along with Kirsty at Medway Asthma Self-Help (MASH). The webinar was a private screening for local Medway football coaches and gave valuable insights on managing, treating and recognising an asthma emergency.
Carol Stonham MBE, has been a registered nurse since 1986, transitioning from acute hospital settings to primary care by 1990. She serves at the Gloucestershire ICB and leads the Respiratory Clinical Programme Group, as well as co-leading the NHSE South West Respiratory Network, Carol is also a member of the Bedfont® Medical Advisory Board.
MASH is a local charity who have been helping the people of Medway since 1996 with information and support for people with asthma. MASH works with GPs, hospital and Asthma UK to raise awareness and knowledge of asthma for the people of Medway.
Children spend a lot of time away from home, whether it is at school, nursery or clubs, therefore, it is important that when they are away from home, they are safe. If a child has asthma, the responsible adults around them need to know what to do in the case of an asthma emergency.
The webinar covers some important topics:
- What asthma is,
- When and how to use an inhaler,
- What to do in an asthma emergency.
How common is asthma?
Asthma is a common respiratory condition that affects around 5.4 million people in the UK1, with 1.1 million of them being children2. Asthma is the most common long-term condition among children and young people and the UK has among the highest mortality rates in Europe. It is thought that emergency admissions and deaths related to asthma are largely preventable, with these statistics being linked with deprivation2.
It is encouraging to see a small reduction in deaths among children and young people as a result of asthma between 2008-20183, however, even though the figures are low, they are still too elevated for a condition that should be manageable, making the deaths preventable.
Unfortunately, the trend seems to be rising among 15 – 24 year olds3. A report in 2019 found that out of 19 countries, young people are more likely to die from asthma in the UK, compared to those in other wealthy countries4. This is not good enough and we should be doing better than that.
What is asthma?
According to the British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines, asthma is the presence of more than 1 of the following symptoms:
- Wheeze,
- Breathlessness,
- Chest tightness,
- Cough.
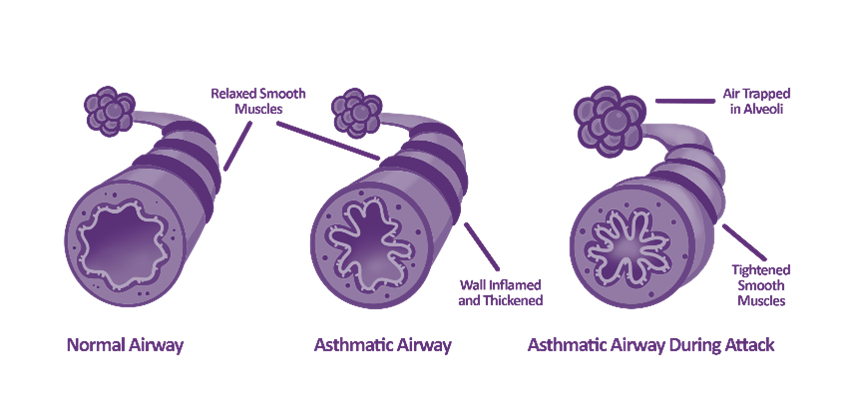
It is a combination of variable symptoms, and you may find that over weeks or months, you experience a difference in symptoms. The underlying problem is the airways, the airways can either have hyperresponsiveness, which is an increased sensitivity of the airways or inflammation.
What symptoms do asthma sufferers typically suffer from?
The most common symptoms of asthma are:
- Cough,
- Wheeze,
- Shortness of breath,
- Exercise limitation,
- Duration of symptoms,
- Triggers.
Hopefully, no one suffers from all of the above, but symptoms are variable and can come and go over a period of time. Quite often the person knows what sets their symptoms off.
How does asthma affect the lungs?
Asthma causes an inflammation of the mucous membrane, which can lead to swelling into the airspace, increase in mucous production, twitch airways and bronchospasms.
How do we measure asthma?
There are a few ways asthma can be measured/monitored. First would be a peak expiratory flow meter, this is a simple test where you have a device with a scale. First, you would make sure the slide on the scale is on 0, you would then take a deep breath in and provide a short, sharp blow into the meter.
Another way is spirometry, which is a more advanced look at how the air moves in and out of the chest. It measures how much air you can move out of your chest and how quickly you can do that, as well as the pattern the air follows as it leaves the chest. This test would be carried out in a clinic.
Finally, there is a Fractional exhaled Nitric Oxide (FeNO) test. As standard, we produce small amounts of Nitric Oxide in our breath, but when a patient has inflammation in the chest, the type typically seen in asthma, more Nitric Oxide is produced. A FeNO test, carried out by the NObreath® is suitable for both adults and children, it requires you to take a deep breath in and blow into the device at a steady pace.
Asthma is a serious, long-term condition, and poorly controlled asthma can present physical symptoms. Symptoms in children can include:
- Shortness of breath,
- Tight chest,
- Wheeze.
These symptoms can create further issues in children, as if they feel unwell, they are less likely to take part in physical activity, this then leads them to become deconditioned and at risk of obesity, which can make asthma worse. Poorly controlled asthma can also disturb sleep, which affects concentration, emotions and behaviour.
There are also psychological effects that poorly controlled asthma can have on children. Children, typically like to be like other children and having asthma can make them feel different. They might be embarrassed by their inhaler, meaning they may not use it when needed. They may have experienced an asthma attack in the past and it might make them frightened to do things that could trigger another attack, limiting them to what they can do.
Poorly controlled asthma can also affect education outcomes, as children with asthma may be absent from school for medical appointments or asthma attacks. They might also have reduced concentration due to disturbed sleep or feeling unwell.
How do we treat asthma?
There are around 119 different inhalers available, so there are lots of treatments and options. Our aim is for anyone with asthma to have:
- No daytime symptoms,
- No night symptoms,
- No activity limitations,
- No need for using a rescue inhaler,
- No side effects from treatment,
- Normal lung function.
The main part of treating asthma is treating the underlying inflammation, and if we get that treatment right, the swelling will settle down, the airways will open back up and the patient will find it easier to breathe. However, not all treatment is instant, if a brown-coloured preventer inhaler is used, it must be used for a few days. This allows the medication to settle down the inflammation slowly and once settled it is likely to be needed long-term.
Inhalers are broken down into 2 categories:
Treatment:
- Treats underlying inflammation,
- Settles swelling,
- No instant effect,
- Long term treatment.
Rescue:
- Treats twitchy airways,
- Relieves symptoms quickly,
- Lasts up to 4 hours,
- Should only be required occasionally.
If anyone is using a blue rescue inhaler more than 3 times a week, their treatment is not quite right and this would need to be assessed. It is important to know the difference between the treatment inhaler, usually orange and brown in colour and the rescue inhaler which is always blue.
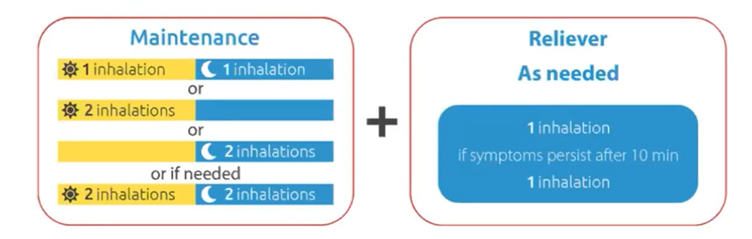
Maintenance and reliever therapy (MART) is also used, this is a combination inhaler. The inhaler contains treatment and rescue medication and is used regularly twice a day if there are symptoms. Extra doses of this inhaler should only be needed occasionally.
It is important to get the inhaler technique right, sadly, many people do not know how to use their inhaler properly and this is partly due to insufficient advice from their healthcare provider. This proves that education is key to enabling patients to take their medication correctly.
Types of inhalers:
- Pressurised metered dose inhaler,
- Spacer devices,
- Breath-actuated meter dose inhaler,
- Dry powder inhaler.
Knowing the different types and the correct way to use them is important. If you have a metered dose inhaler, the breathing technique should be slow and gentle, if you have a dry powder inhaler, you should breathe in fast and hard. If you use the fast and hard technique with a metered dose inhaler, the medication will hit your throat rather than go into your lungs, likewise with the dry powder inhalers, if you do not breathe in fast and hard, you will not move the powder into the lungs.
Spacers are available, usually for children. They are chambers for metered dose inhalers, to slow the speed of the medication and hold it for a few seconds, allowing more time for the user to breathe in. There are many different sizes of spacers available, depending on the age and capability of the users.
What if my asthma is not that good?
If you have all of the previous steps in place and your treatment is correct, asthma can still flair up. If this happens, there are steps you can take to tackle this.
- Use treatment inhalers regularly,
- Make sure you know how to best use your inhaler,
- Be aware of triggers,
- Book a review with the asthma nurse,
- If you need your blue rescue inhaler more than 3 times a week, book an appointment,
- If you are short of breath and your inhaler is not helping, make an urgent GP appointment for the same day.
What should you do in an asthma attack?
Knowing what to do during an asthma attack can be the difference between life and death, the below graphic from Asthma + Lung UK shows the steps you should take during an attack.
https://www.asthmaandlung.org.uk/conditions/asthma/asthma-attacks#:~:text=an%20AIR%20inhaler-,Sit%20up%20%2D%20try%20to%20keep%20calm.,not%20improving%2C%20repeat%20step%202
To ensure the care is right, everyone should have a personalised asthma action plan. Various formats are available, an example from Asthma + Lung UK below is:
Action plan available from https://www.asthmaandlung.org.uk/conditions/asthma/manage/your-asthma-action-plan
The green area represents when your asthma is good, this is how it feels when my asthma is good and this is the medication I take to maintain good asthma control.
Amber is when things are starting to get worse, how can I recognise when it is beginning to deteriorate? It gives you steps to follow to improve symptoms and move back into the green.
Red represents an asthma emergency and what to do in this situation.
It is important to recognise when you are in the red and what to do in case this happens, but it is also good to realise when you are in the amber so you can get yourself back on track to green.
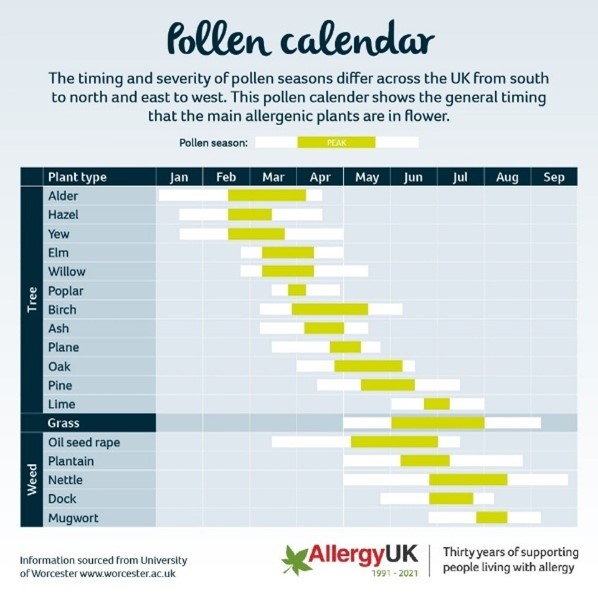
Seasonal Variations
here are seasonal variations where we see peaks in asthma admissions, and these can be:
- Autumn – Back to school,
- Winter – Winter colds,
- Spring – Pollen.
The pollen calendar for the UK shows peaks not just in spring but throughout the year
What can trigger asthma?
There are various triggers for asthma, but the most common are:
- Exercise,
- Bugs in the home,
- Chemical fumes,
- Cold air,
- Fungus spores,
- Dust,
- Smoke,
- Strong odours,
- Pollution,
- Anger,
- Stress,
- Pets.
With some lesser-known triggers, such as:
- Hormones,
- Damp and mould,
- Infection,
- Change in temperature,
- Thunderstorms,
- Foods,
- Alcohol,
- Drugs,
- Sex
How can we manage the effect of our triggers?
Sometimes, there is not much that can be done to avoid our triggers, but where possible, you can eliminate or avoid them. You can treat other conditions that trigger your asthma and make sure your asthma is well managed. You can predict the triggers. For example, if hay fever is a trigger, start treating your hay fever early. Lastly, always have your rescue medication to hand.
Parents and carers must understand asthma to understand how the medication works and why it is important. They need to have an asthma action plan, know how to maintain control and know what to do in an emergency. They also need to know what to do if an inhaler has been forgotten.
With asthma still an ongoing serious respiratory condition that kills children every year, this webinar has helped people understand the following:
- Asthma and the treatment,
- The goals of the treatment,
- How and when to use inhalers,
- Why asthma worsens,
- What to do in an emergency.
To watch the full webinar and gain in-depth insights, click here.
References
- Asthma + Lung UK. [cited on 8/10/24] Available from https://www.asthmaandlung.org.uk/conditions/asthma/what-asthma
- RCPCH State of Child Health. [cited on 8/10/24] Available from https://stateofchildhealth.rcpch.ac.uk/evidence/long-term-conditions/asthma/
- PMC PubMed Central. Wei-Yu Chen, Ching-Wei Lin, Ju Lee, Po-Sung Chen, Hui-Ju Tsai and Jiu-Yao Wang. [cited on 8/10/24] Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8102795/
- BBC News. [cited on 8/10/24] Available from https://www.bbc.co.uk/news/health-47292157#:~:text=Young%20people%20in%20the%20UK%20are%20more%20likely%20to%20die,of%2019%20high%2Dincome%20countries.