Research has shown that seven out of ten people with asthma report that cold air exacerbates their asthma symptoms1. As winter approaches quickly, it is essential to prepare for asthma management in advance. A vital tool in asthma management, as recommended by the National Institute for Health and Care Excellence (NICE), the British Thoracic Society (BTS), and the Scottish Intercollegiate Guidelines Network (SIGN), is Fractional exhaled Nitric Oxide (FeNO) testing2. In this blog, we will explore how FeNO testing can play a crucial role in asthma management this winter.
Winter challenges for asthma patients
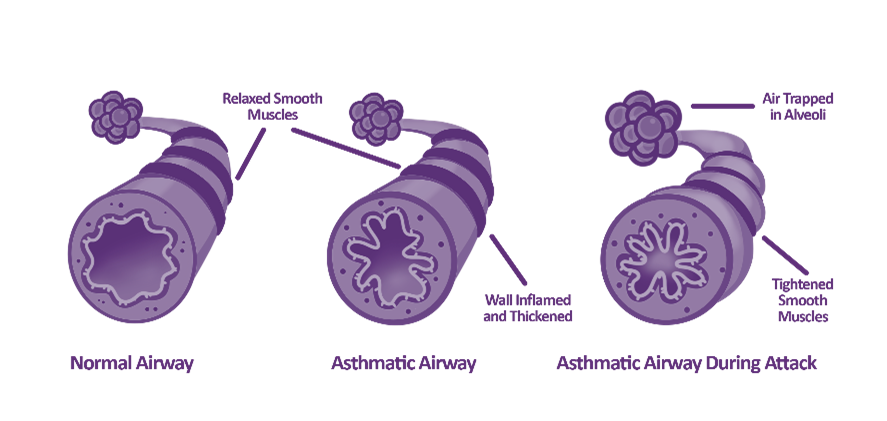
During the winter months, the air temperature drops, and this can impact people living with asthma for various reasons:
- Cold air causes the airways to narrow1.
- Cold, dry air can irritate the airways, exacerbating symptoms1.
- Cold air can weaken the immune system, making it easier to catch respiratory infections1.
- Spending more time indoors during winter makes the spread of respiratory infections easier1.

Asthma + Lung UK found that hospital admissions for lung diseases, such as asthma, rose by 80% in December, January, and February, compared to Spring3. These figures highlight the significant impact that respiratory conditions, such as asthma, have on healthcare systems during the winter months. FeNO testing is a vital tool in improving the quality of life for asthma patients during the winter months.
What is FeNO testing?
FeNO is a biomarker for eosinophilic airway inflammation, a condition commonly associated with asthma. When a person’s airways are inflamed, more Nitric Oxide (NO) is produced, and this can be measured in exhaled breath in parts per billion (ppb). Taking a FeNO test with a FeNO device like the NObreath® is a quick, easy, and non-invasive process. During the test, the patient takes a deep breath and then exhales into the NObreath®. An instant FeNO reading is displayed, allowing you to determine a person’s level of airway inflammation in as little as five minutes4.
FeNO testing with the NObreath®
The NObreath® is an innovative FeNO device, specifically designed for use in primary and secondary care settings. Fully portable, the NObreath® can be easily carried between consulting rooms, providing a quick and convenient solution for FeNO testing in busy settings. The device has an adult and child test mode, making it perfect for all ages.

Why is FeNO testing crucial before and during winter?
Providing a FeNO test at regular asthma reviews allows clinicians to proactively monitor a patient’s airway inflammation, allowing them to identify rising inflammation before symptoms spike. It also helps to provide personalised treatment plans and guide inhaled corticosteroid (ICS) titration, ultimately reducing exacerbations and unnecessary hospital visits.
The latest joint UK guidelines from NICE, BTS, and SIGN recommend FeNO as a first-line test for asthma diagnosis and a vital tool for asthma management2, making it clear that FeNO testing should be available throughout the country. With this in mind, regular asthma reviews are essential throughout the year, not just during the winter months. FeNO testing is the perfect way to establish a person’s airway inflammation, allowing healthcare professionals to personalise treatment plans and therefore improving quality of life for those with asthma.
For more information on FeNO testing with the NObreath®, visit the website here.
References
- Asthma and Lung UK. Cold Weather and Your Lungs | Asthma + Lung UK [Internet]. www.asthmaandlung.org.uk. 2023. Available from: https://www.asthmaandlung.org.uk/living-with/cold-weather
- NICE. Overview | Asthma: diagnosis, monitoring and chronic asthma management (BTS, NICE, SIGN) | Guidance | NICE [Internet]. Nice.org.uk. NICE; 2024. Available from: https://www.nice.org.uk/guidance/NG245
- Out in the cold: lung disease, the hidden driver of NHS winter pressure | Asthma + Lung UK [Internet]. www.asthmaandlung.org.uk. Available from: https://www.asthmaandlung.org.uk/out-cold-lung-disease-hidden-driver-nhs-winter-pressure
- Fractional Exhaled Nitric Oxide (FeNO) Test | North Bristol NHS Trust [Internet]. Nbt.nhs.uk. 2024 [cited 2025 Jun 23]. Available from: https://www.nbt.nhs.uk/our-services/a-z-services/respiratory-medicine/respiratory-patient-information/fractional-exhaled-nitric-oxide-feno-test