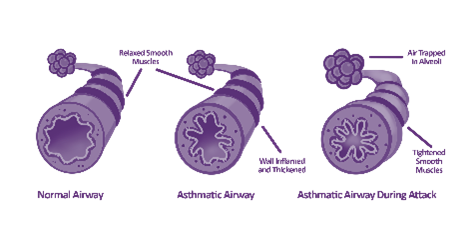
The latest UK asthma guidelines1 have identified how Fractional exhaled Nitric Oxide (FeNO) testing fits into the diagnosis and ongoing management of asthma. The guidelines are based on the latest clinical evidence, cost-effectiveness, and cost-effective modelling, so they are strongly recommended for use. But what does that mean for the individual patient as they travel along their asthma journey? Let’s consider a patient.
Meet Ramila. She is 19 and presents to her GP with symptoms of chest tightness, wheeze, and shortness of breath. Initially, it was only related to exercise, but now it happens more often, and Ramila notices it, especially when she wakes in the morning. It is not constant, so Ramila has been putting off seeing her GP.
Ramila remembers having an inhaler, a blue one, she thinks when she was younger, but has never had a formal diagnosis of asthma. Her older brother has asthma, as does her aunt. Ramila has never had hay fever or eczema symptoms, has no allergies, and is otherwise well.
The GP listens to Ramila’s chest, which is normal. This does not rule out asthma when she is asymptomatic. As Ramila’s GP has access to FeNO within the practice, they can perform a FeNO test at this appointment. The result is 61 parts per billion (ppb), which, given the history and symptoms Ramila describes, supports a diagnosis of asthma. The latest guideline recommendation is that this single test is enough to confirm a diagnosis of asthma, and Ramila can begin treatment without delay.
Had the GP not had access to FeNO or had there been a delay in accessing the test, they could have ordered a blood test to look at Ramila’s level of eosinophils. This would mean there would be a delay in diagnosis, and potentially in treatment, whilst the test was processed, as this is not a near-patient test. It would likely have also resulted in Ramila having to make another appointment, putting extra pressure on primary care services and her busy schedule.
Once asthma is diagnosed, Ramila can start on a treatment regimen. If she is having occasional symptoms, then treatment with a Formoterol-containing inhaled steroid combination inhaler used on an as-needed basis is recommended. This is an Anti-Inflammatory Reliever (AIR) regimen. As Ramila is having daily symptoms, it would be appropriate to move to a Maintenance And Reliever Therapy (MART) regimen. This would still involve the initiation of a Formoterol-containing inhaled steroid combination inhaler, but used regularly each morning and evening, and on an as-needed basis for symptom control. A short-acting bronchodilator is not indicated in the AIR or MART regimen. The first line choice of device type should be a dry powder if Ramila can use it. This has a much lower carbon footprint.
Ramila would need to be shown how to use the inhaler device correctly, have some education regarding what asthma is and how the medication works, and start to produce a personalised asthma action plan with the clinician. This will help Ramila to know what good asthma control looks like and what medication she should take to achieve this. It will also help her recognise any deterioration in her symptom control and when things need urgent advice. The plan should tell Ramila whom to contact, when her symptoms should be less well controlled, and what to do in the more urgent situation.
This is also a good opportunity for more general health advice around smoking, alcohol, weight management and maintaining an active lifestyle.
Ramila will be invited to attend regular reviews. The guidelines suggest this as a good time to check a FeNO level. It is an opportunity to reinforce patient education, and FeNO is a good tool to demonstrate whether the current medication regimen controls airway inflammation or not. If the FeNO result is raised, it opens a dialogue around how well Ramila understands the diagnosis of asthma and how her prescribed inhalers work. Ramila has her FeNO checked, and it has crept up to 52 ppb, having previously been settled around 18 ppb. After discussion, it became clear Ramila has become more complacent about her asthma and is using her inhaler less regularly and had been using it just as she needed it, but on reflection, she could see that her symptoms were not as well controlled as they had been. FeNO is a good tool to uncover nonadherence with medication and open the conversation in a non-judgmental way.
The guidelines also suggest checking FeNO before and after changing medication. It can help decide what treatment might be appropriate and help assess the response to treatment. This is particularly pertinent when Ramila attends with poor asthma control a few years later. She has good inhaler technique and has been using her inhaler with a MART regimen with good adherence. Despite this, her symptoms are increasing. She had been increased from a low-dose MART regimen to a moderate-dose MART regimen at a previous review (increasing the steroid dose of the combination inhaler), which had been controlling her symptoms for some time until recently. In this instance, the guidelines suggest checking FeNO to guide the next treatment decision. Ramila was found to have a normal FeNO (21 ppb), but with increasing symptoms, will need an increase in treatment. With a normal FeNO (indicating that eosinophilic airway inflammation is well controlled), the clinician is guided to add additional treatment. This could be Montelukast, taken orally once a day, in addition to the MART regimen, or the addition of a Long-Acting Muscarinic Antagonist (LAMA) as inhaled therapy. The options, advantages and potential side effects were discussed with Ramila, who opted to try Montelukast.
Had Ramila demonstrated a raised FeNO, the treatment recommendation would have been directed towards further anti-inflammatory therapy. The options here are to prescribe high-dose inhaled steroids or initiate biologic therapy. Both options would be considered, and decisions would be made with Ramila and a respiratory physician, so Ramila would need a referral for this. Without FeNO to guide decisions, a referral could be unnecessarily delayed.
It is also worth considering how we reduce asthma therapy, asthma is a variable condition, so treatment needs to be titrated up and down at times of variation. Had Ramila been on a moderate dose MART regime and been asymptomatic for at least 3 months, not experienced an exacerbation and not needing to use additional rescue doses of medication, all indicating good symptom control, it would be worth discussing stepping back to a low dose MART regime, reducing the steroid component on the combination inhaler. FeNO is a useful tool to guide this process if the person fits the criteria for good control. If Ramila’s FeNO test result was also low, indicating that eosinophilic airway inflammation was well controlled, it would be a good time to discuss reducing the steroid dose, provided she was not approaching a known trigger time of year. However, stepping the inhaled steroid dose would not be wise if it did not indicate good inflammation control in that it was even moderately raised.
Although the associations seem to sit around using FeNO in diagnosing asthma, the latest iteration of the UK guidelines demonstrates the importance of FeNO testing as part of the whole asthma patient pathway. Easy access without delay will enhance the accuracy of care delivered to people presenting with asthma in primary care.
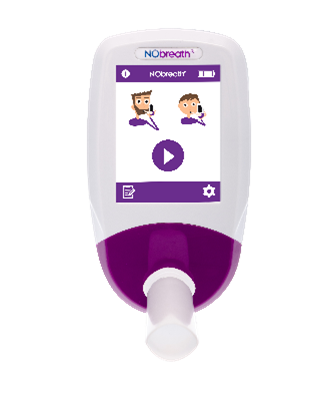
FeNO testing with the NObreath®:
Bedfont® Scientific Limited, are world leaders in breath analysis, with over 48 years of expertise and knowledge in designing and manufacturing breath analysis devices. Bedfont® are committed to improving patient safety through innovating breath analysis devices, such as the NObreath®. The device is a portable handheld FeNO device, used by healthcare professionals to aid in the diagnosis and treatment of asthma.
For more information on the NObreath® and FeNO testing, visit the NObreath® website.
References:
- National Institute for Health ad Care Excellence (2024) Asthma: diagnosis monitoring and chronic asthma management (BTS, NICE, SIGN). Available from https://www.nice.org.uk/guidance/ng245 [Last accessed 11.4.2025]