
Products
Bedfont® have a wide range of breath analysis devices which all have unique uses. Our expertise is reflected in the wide range of products that we offer – from portable to benchtop devices – which can be used to analyse the breath of patients.
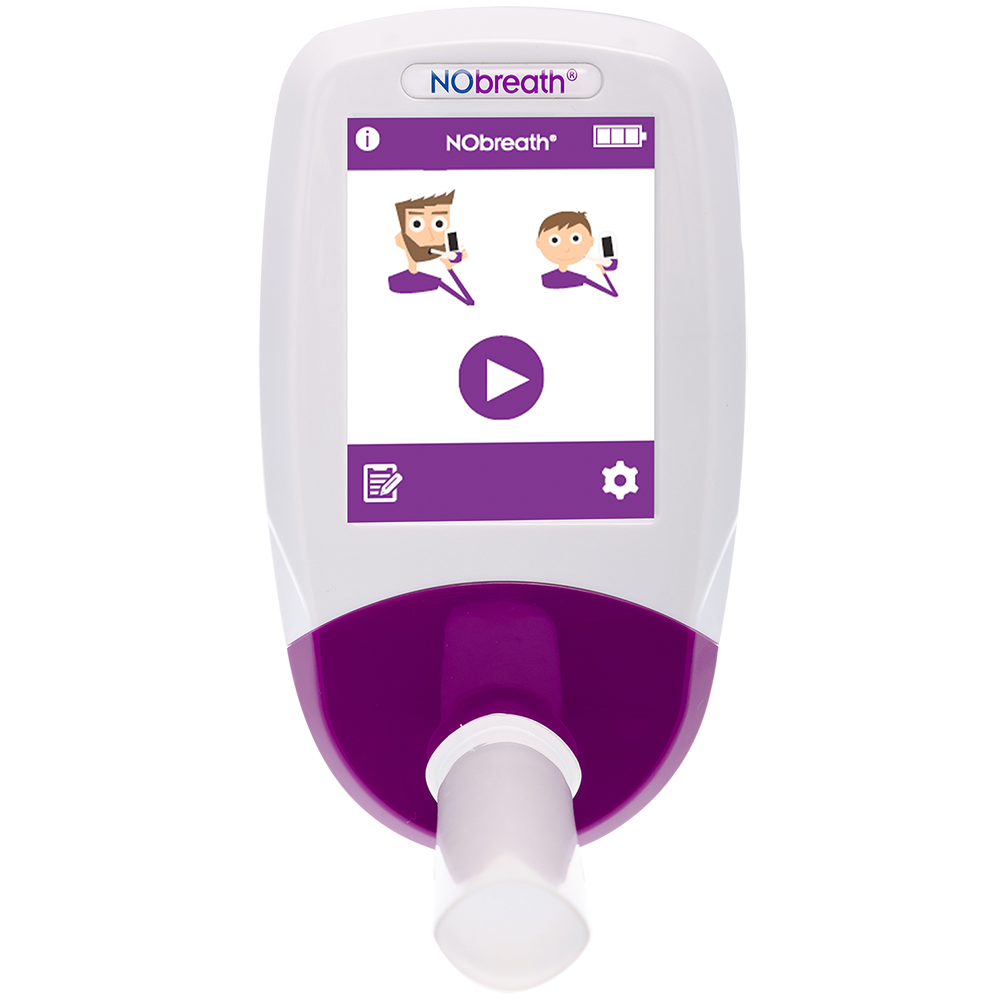


NObreath®
The NObreath is a FeNO device which can be used to measure airway inflammation for the diagnosis and management of asthma. Conforms to BTS/NICE/SIGN, ATS & ERS guidelines, the NObreath makes FeNO measuring quick and easy, plus it is completely non-invasive, with the ability to monitor airway inflammation in both adult and child patients. Furthermore, the ambient monitoring mode enables you to check ambient levels of NO.

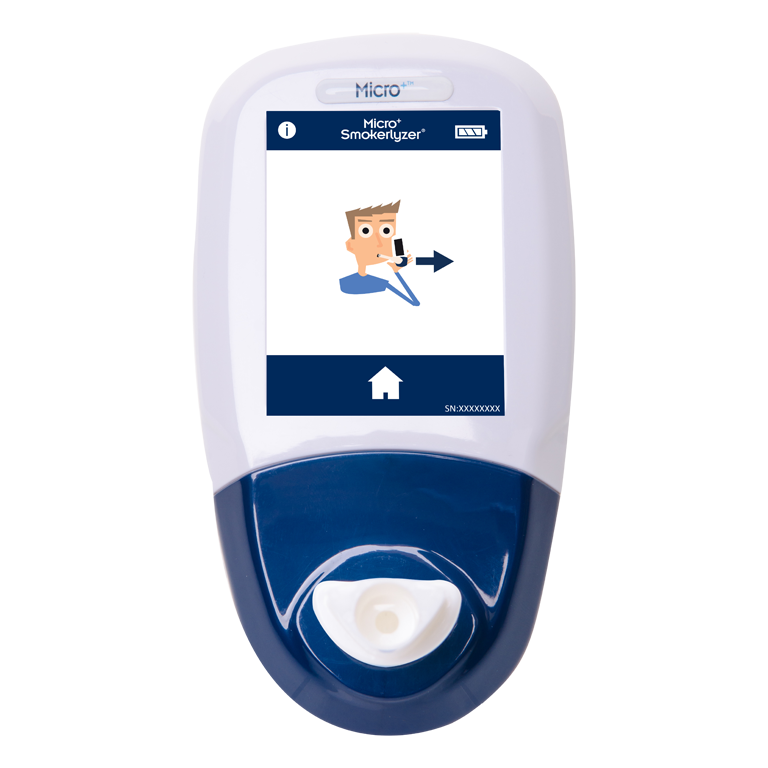

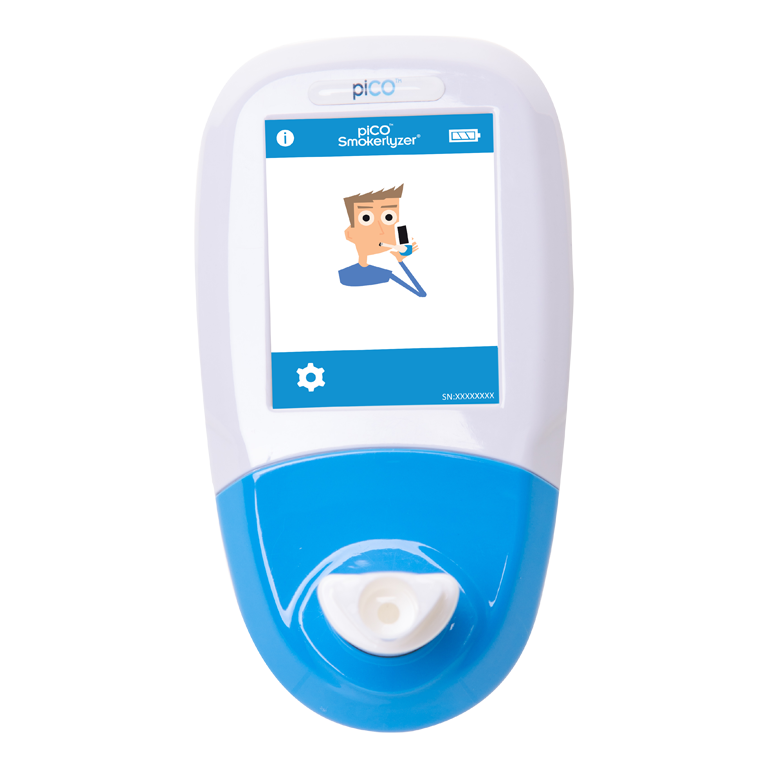

Smokerlyzer®
The products in the Smokerlyzer range are all breath carbon monoxide (CO) devices to aid smoking cessation. The devices measure the amount of CO on a smoker’s breath. For professionals, it is a way to biochemically establish a person’s smoking status and for the smokers themselves, the Smokerlyzer is a motivational visual aid to encourage them to quit and to measure their progress whilst doing so.



Gastrolyzer®
Helping to detect gastrointestinal disorders, the Gastrolyzer® range offers accurate hydrogen breath testing.
The Gastrolyzer range is a set of medical devices designed to measure various gases in the breath of patients for the purposes of diagnosis and treatment of various gastrointestinal (GI) disorders.

Medi-Gas Check
The Medi-Gas Check range is a suite of fixed and portable gas detection monitors used to verify and validate the quality and quantity of medical gas administered to patients, from the plant room and distribution points to the point of delivery to the patient. The range conforms fully with the requirements of UK Government Guidance in the form of the Health Technical Memorandum (HTM) 02-01 and other relevant legislation.



CELLER8 generates Pulsed Electromagnetic Fields (PEMF) up to three feet (90cm) from the device giving you full body and localised coverage.
CELLER8 technology also puts you in control of the fundamentals of PEMF therapy including:
- Intensity
- Frequency
- Polarity


The Breathacise spirometer is an incentive spirometer, utilizing a Basketball Game to exercise and help improve your respiratory capacity and strength. It does this by helping you exercise your respiratory capacity through deep breathing.
The Breathacise has a built-in basketball game to provide an incentive for respiratory exercise. Set the basketball hoop to your desired target, inhale and using the indicator to ensure a steady, deep inhalation until you dunk the ball into the hoop.

Software Development Kits
Unlock seamless bio-validation integration with Bedfont SDK’s! Designed for developers who want to bring accurate health monitoring and real-time data to their apps, our SDKs offer a direct link to Bedfont’s trusted medical devices. Whether you’re building an app for asthma management, smoking cessation, or personalised wellness, our SDKs provide secure, compliant, and straightforward connectivity. Give your users the power of clinical – grade insights with just a few lines of code – turn your app into a health solution today!
