Part 3: Asthma treatment in 2024: Navigating the new recommendations for every age
After exploring diagnostic changes and the pivotal role of Fractional exhaled Nitric Oxide (FeNO) in asthma care, part 3 of our series shifts the focus to treatment and long-term management. From the latest recommendations for patients aged 12 and over, as well as tailored treatment pathways for children under 5, this blog summarises practical, guideline-driven approaches for managing asthma more effectively.
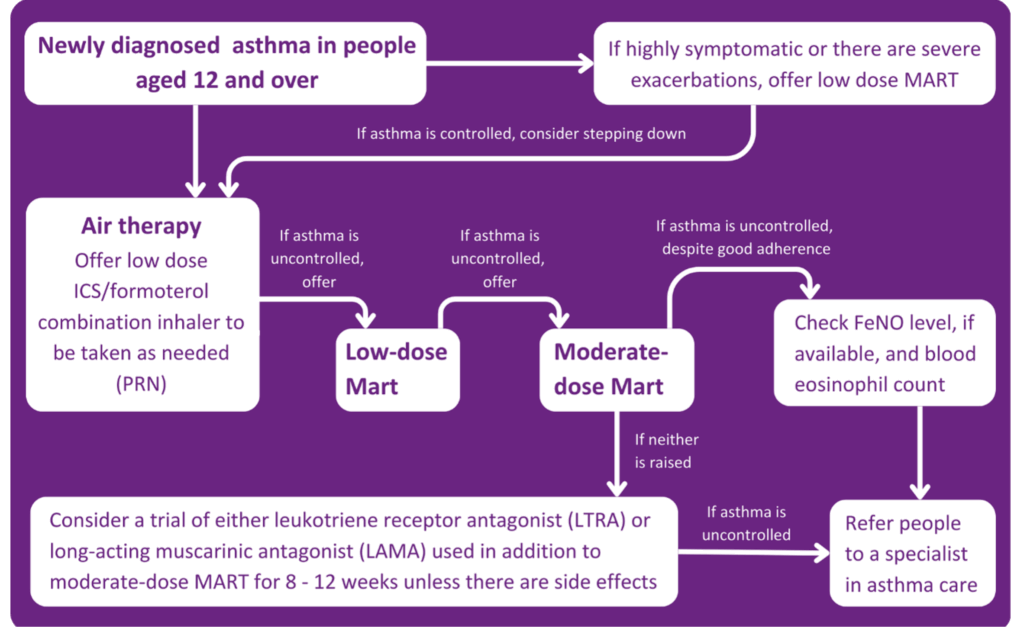
Treatment for patients ages 12 and over
In the past, patients with asthma have always been told to carry their blue inhaler, as this will keep them safe in the event of an asthma attack. This is no longer the case in the new guidelines.
- It is now recommended that people aged 12 and above with a new diagnosis of asthma should be offered Anti-Inflammatory Relief (AIR) therapy. This means using a formoterol-containing inhaled steroid inhaler on an as-needed basis. This process is for someone not experiencing symptoms at the time of presentation.
- If a patient is unwell or exacerbating, this step should be skipped, and the patient should be offered low-dose Maintenance and Reliever Therapy (MART). MART is an asthma treatment plan in which one combination inhaler is used instead of two separate preventer and reliever inhalers.
- If a patient is still symptomatic after being on a low-dose MART regime, then a moderate-dose MART regime should be offered. If this doesn’t relieve symptoms, inhaler technique, adherence, and new triggers should be assessed.
At this point, if a person is still symptomatic, it is important to understand what is causing the symptoms. Is it uncontrolled eosinophilic airway inflammation, or is it that the inflammation is controlled and it is bronchospasm? A FeNO test will measure the patient’s FeNO level. If it is raised, a referral to an asthma care specialist is required. If the FeNO level isn’t raised, the patient should be offered either a Leukotriene Receptor Antagonist (LTRA) or a Long-Acting Muscarinic Antagonist (LAMA) on top of a moderate-dose MART regimen for 8-12 weeks.
If at the end of the 8-12 weeks:
- The asthma is controlled, continue with the treatment.
- If this control has improved but still not completely, continue the treatment or add the LTRA or LAMA (Whichever is not already being taken).
- If the control hasn’t improved, stop the LTRA or LAMA being taken and replace it with the alternative.
If, after these steps, the asthma remains uncontrolled, the patient should be referred to a specialist.
For patients with an existing asthma diagnosis and currently on treatment recommended in the previous guideline, nothing needs to change as long as the asthma is controlled and not much salbutamol is being used. If, however, the patient is only using salbutamol with no other inhalers, it should be considered to push them over to an AIR regime.
- If patients on a low-dose inhaled steroid and not using a MART regimen become symptomatic, they should be moved to a low-dose MART regimen, and if on a moderate-dose steroid, then to a moderate-dose MART regimen.
- If the patient is still experiencing symptoms after this, they should be referred to a specialist. If you have patients on a high-dose steroid and they are symptomatic, they should also be referred.
PCRS – New asthma guidelines infographic. Available from: https://www.pcrs-uk.org/sites/default/files/resource/New_asthma_guidelines_first_steps.pdf
The guidelines also state that before any medication is adjusted or changed, you must address the possible reasons why the asthma is uncontrolled. For example:
- Alternative diagnosis or comorbidities,
- Suboptimal adherence,
- Inhaler technique,
- Active or passive smoking,
- Psychosocial factors,
- Seasonal factors,
- Environmental factors.
Treatment for children (Aged 5-11 years)

PCRS PCRS – New asthma guidelines infographic. Available from: https://www.pcrs-uk.org/sites/default/files/resource/New_asthma_guidelines_first_steps.pdf
Currently, no inhalers are licenced for MART in children, even though the guidelines include MART for consideration in treatment. Just because the licensing isn’t available now doesn’t mean MART cannot be used for children; it can be prescribed off-label. In this case, it should be documented that medication is being used off-label under the current guidelines by NICE/BTS/SIGN 2024.
Treatment for children under 5

PCRS – New asthma guidelines infographic. Available from: https://www.pcrs-uk.org/sites/default/files/resource/New_asthma_guidelines_first_steps.pdf
What about QOF?
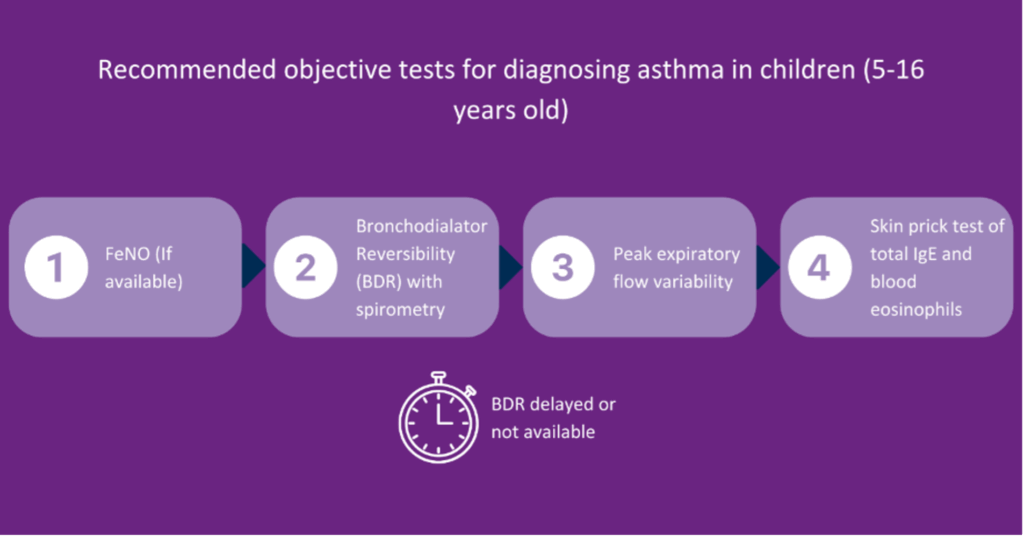
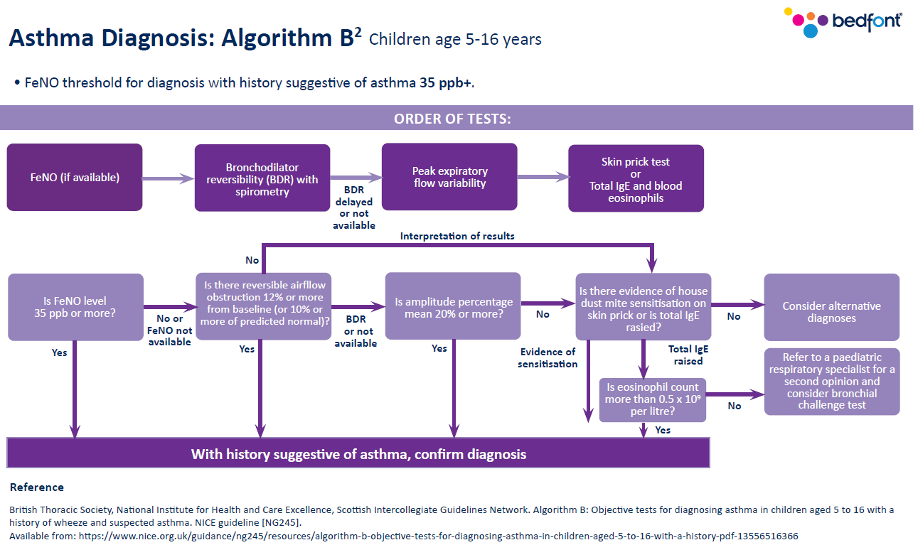
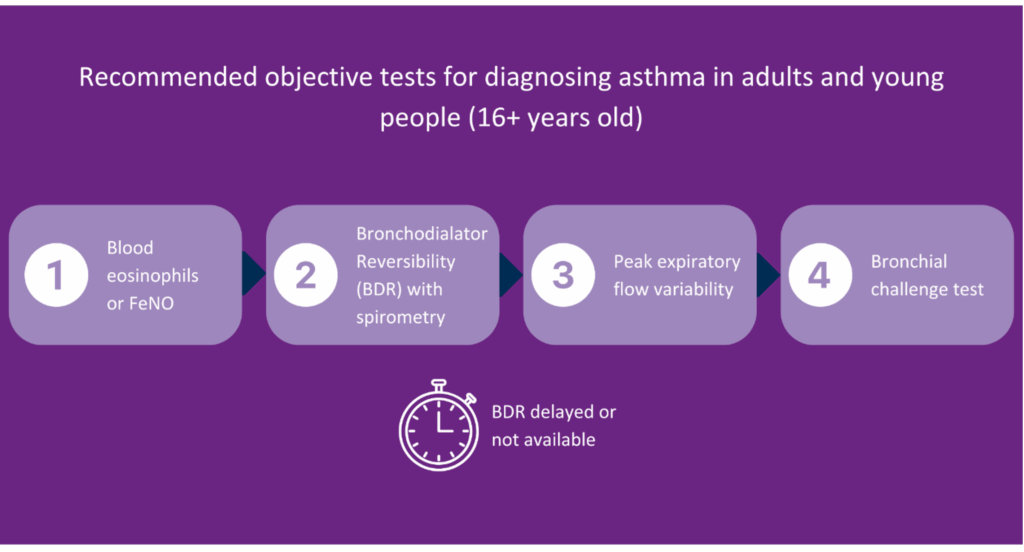
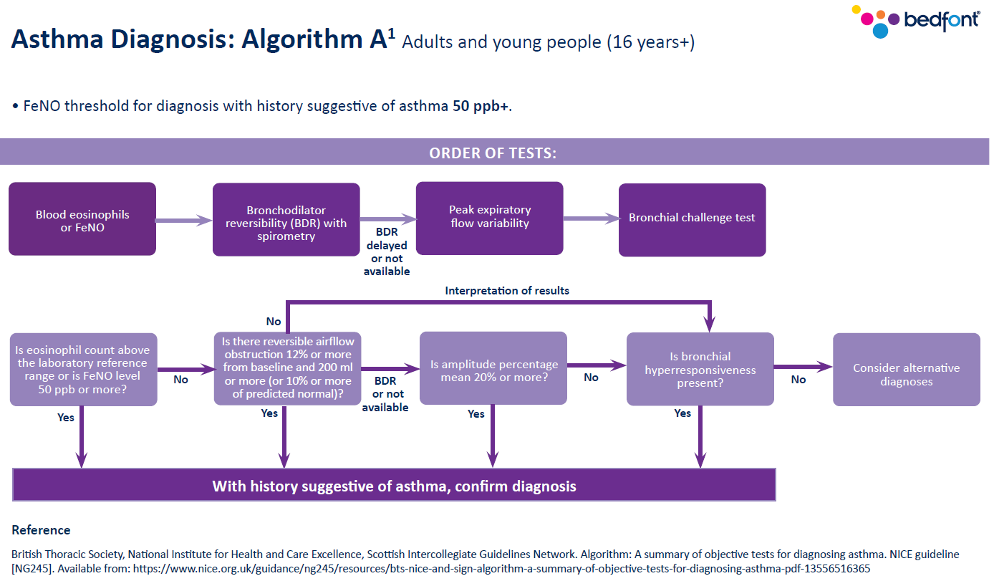
Previously, the Quality and Outcomes Framework (QOF) said spirometry and one other test should be conducted; however, that has now changed in line with the current guidance. You should now look at the number of patients newly diagnosed with asthma who have had one of the following tests:
- Eosinophil count,
- FeNO,
- Spirometry,
- Peak flow with Bronchodilator Response (BDR),
- Bronchial responsiveness (In adults),
- Skin prick test or blood IgE (In children).
This is between 3 months before or 3 months after diagnosis. The diagnosis must be coded correctly to qualify for QOF points.
Monitoring asthma
At every asthma review, asthma control should be monitored by asking the following questions:
- Has there been any absence from work or school due to asthma?
- How much reliever inhaler is being used, including a check of the prescription record.
- The number of courses of oral corticosteroids.
- Have there been any hospital admissions or emergency visits due to asthma?
The guidelines recommend using an asthma control test at reviews and say not to use peak flow to assess asthma control unless there is a person-specific reason to do so. FeNO testing should also be considered in reviews for adults and before and after changing asthma medication.
Poor asthma control
If a patient has poor asthma control, it is recommended to check their FeNO level, as this may indicate poor adherence to treatment or the need for an increased dose of Inhaled Corticosteroids (ICS).
A Short-Acting Beta2 Agonist (SABA) should no longer be prescribed without an ICS, and if any change to asthma medication is made, you should review the response within 8-12 weeks.
Changing medication
There is now also guidance on what you should base the choice of inhalers on. The following must be considered:
- Can the patient use the inhaler correctly?
- The patient’s preference for inhalers.
- The lowest environmental impact among suitable devices.
- The presence of a dose counter.
It is also noted that a spacer should be prescribed with a metered dose inhaler, particularly in children.
The inhaler technique must be checked at every asthma review when control deteriorates and if the inhaler changes. A suitable alternative should be found if the patient cannot use the device correctly.
Risk care
HCPs should consider identifying asthma patients who are more at risk of poor outcomes. Risk factors to consider are:
- Non-adherence to medication.
- Overuse of SABA inhalers (More than two a year).
- Needing two or more courses of corticosteroids per year.
- Two or more emergency or hospital admissions for asthma per year.
This is just a recommendation to evaluate those who are more at risk; you should not forget about the other patients.
The latest guidelines from NICE, BTS, and SIGN introduce significant changes to the diagnosis and management of asthma. While adapting to these updates may initially be challenging, proper education and resources will help healthcare professionals integrate them effectively. In the long run, this unified approach will enhance asthma care for thousands of patients and improve outcomes.
To watch the full webinar, ‘Practical Insights for Asthma Care. The New NICE/BTS/SIGN Guidelines. Why FeNO First?’, click here.