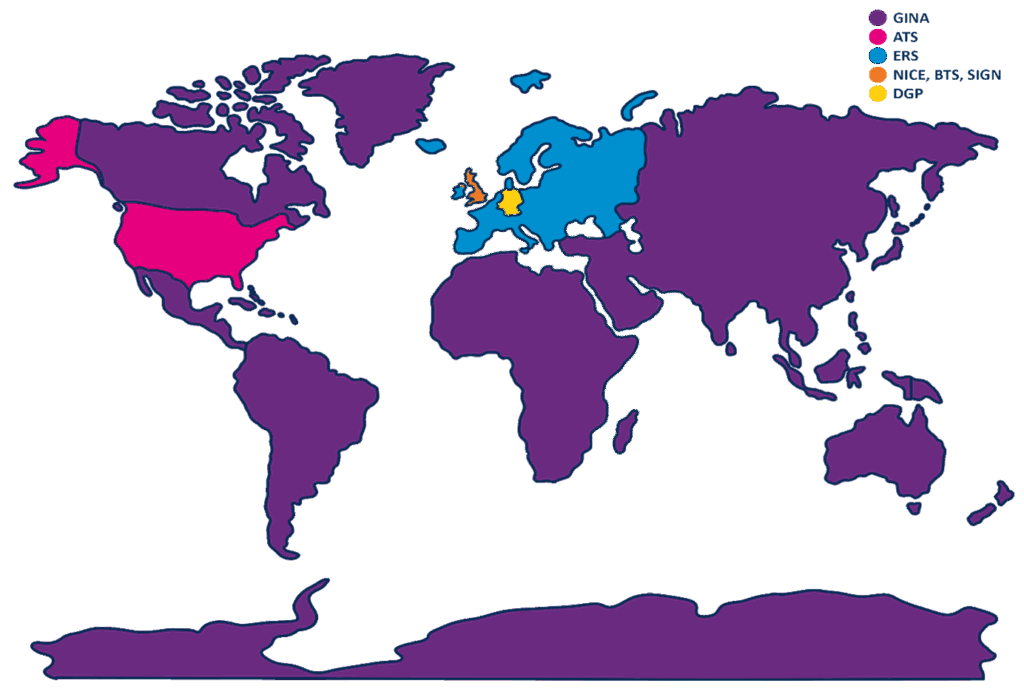
Fractional exhaled Nitric Oxide (FeNO) is extensively utilised in both primary and secondary care settings worldwide. Many regions recommend FeNO-guided management as part of their clinical protocols. This article reviews FeNO-related guidelines in the UK and internationally, with a focus on comparing approaches across different regions.
Guidelines
NICE, BTS, and SIGN guidelines1:
The latest and most significant updates to UK guidelines were in November 2024, when the National Institute for Health and Care Excellence (NICE), the British Thoracic Society (BTS), and the Scottish Intercollegiate Guidelines Network (SIGN) updated and published a joint guideline on asthma diagnosis, monitoring, and chronic asthma management. Before this, NICE, BTS, and SIGN published their guidelines independently, the newly published guidelines bring harmonisation across the board. This review brings significant changes to asthma care approaches, including applying FeNO testing- an objective airway inflammation test for aiding in asthma diagnosis and management.
The guideline can be read here.
ERS guidelines2:
In 2022, the European Respiratory Society (ERS) updated its guidelines for the diagnosis of asthma in adults. In patients suspected of asthma, in whom the diagnosis is not established based on the initial spirometry combined with bronchodilator reversibility testing, ERS suggest measuring FeNO as part of the diagnostic work-up of adults aged > 18 years old with suspected asthma (conditional recommendation for the intervention, moderate quality of evidence).
The guideline can be read here.
DGP guidelines3:
The German Respiratory Society (The Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin, DGP) is the largest and oldest medical professional organisation for respiratory disorders. The latest guidelines on asthma, published in 2023, titled ‘S2K guidelines for specialist diagnosis and therapy of asthma’. FeNO is described as an indispensable component of specialist asthma diagnostics.
The guideline can be read here.
ATS guidelines4,5:
In 2011, the American Thoracic Society (ATS) developed guidance on the interpretation of FeNO testing in adults and children (up to 12 years old). The latest update to the American Thoracic Society (ATS) regarding FeNO to guide the treatment of asthma was in 2021. The update includes FeNO testing being strongly recommended to manage asthma in patients, in addition to usual care.
The American College of Allergy, Asthma and Immunology (ACAAI) and the American Academy of Allergy, Asthma and Immunology (AAAAI), published a joint statement in 2012 in response to the ATS guidelines “The American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology formally recognize and support the 2011 ATS Clinical Practice Guideline on the Interpretation of Exhaled Nitric Oxide for Clinical Applications.”6.
The guideline can be read here and here.
GINA guidelines7,8:
The Global Initiative for Asthma (GINA) works with healthcare professionals, patient representatives, and public health officials around the world to reduce asthma prevalence, morbidity, and mortality. GINA’s guidelines, which were updated in 2024, recognise FeNO as a useful biomarker for aiding in asthma diagnosis and management. The guideline provides ppb recommendations for diagnosis; however, for children, GINA does not specify exact cutoffs but acknowledges its role in guiding treatment.
The guideline can be read here and here.
Reimbursement for FeNO:
Each country has different policies regarding reimbursement for FeNO testing. In England, practices strive to accomplish maximal Quality and Outcomes Framework (QoF)9 points to maintain practice income and fund expenses such as the purchase and maintenance of equipment, for example, FeNO devices. Currently, the QoF requirement for diagnosis of asthma is spirometry and one other test, such as FeNO, bronchodilator reversibility or measures of variability. With the change in the BTS/SIGN/NICE guideline, this will change in line with the guideline recommendations, with the requirement that practices perform at least one objective test that indicates asthma. In adults initially, this could be FeNO or blood eosinophils; in children, the initial test must be FeNO.
Across Europe, reimbursement policies vary widely, depending on national health guidelines. In Germany, FeNO testing is endorsed in the national asthma guidelines; however, FeNO is not reimbursed by Statutory Health Insurance (SHI) in primary care settings. In North America, Medicare and Medicare Advantage plans provide reimbursement for FeNO testing if the test is deemed medically necessary by your healthcare professional10.
Why guidelines matter:
The use of asthma diagnosis and management guidelines in the application of FeNO is essential globally, to ensure standardised, evidence-based asthma management, tailored to varying healthcare infrastructures and patient demographics. These guidelines empower healthcare professionals to make informed decisions, enhancing the accuracy of asthma diagnosis and treatment. Established guidelines fosters consistency in care, contributing to sustainable healthcare costs, reducing misdiagnosis, and ultimately improving patient outcomes. It’s worth noting that besides the countries listed in this article, numerous others also have national guidelines, including Japan, Italy, China, France, Mexico, Spain, Malaysia, Australia, and many more!
Key Takeaways
NICE, BTS, SIGN guidelines1:
Diagnosis:
- For adults, asthma can be diagnosed if FeNO levels are ≥ 50 ppb or higher, an increase from the previous NICE guideline’s 40 ppb or higher threshold.
- For children, asthma can be diagnosed if FeNO levels are ≥ 35 ppb or higher. This has remained the same as the previous NICE guidelines.
- FeNO testing is recommended as first-line testing in asthma diagnosis for adults and children.
- If the first test is diagnostic, further diagnostic testing is not required.
Management:
- FeNO testing has been acknowledged as a tool in asthma management.
- It aids to inform healthcare professionals when changing or adjusting asthma therapy.
- Recommend FeNO use for asthma monitoring in adults.
ERS guidelines2:
Diagnosis:
- A cut-off of 40 ppb offers the best compromise between sensitivity and specificity while a cut-off of 50 ppb has a high specificity of > 90% and is therefore supportive of asthma diagnosis.
- A FeNO value < 40 ppb does not rule out asthma, and similarly, high FeNO levels themselves do not define asthma.
Management:
- Measure FeNO as part of the diagnostic work-up of adults aged 18 years with suspected asthma (conditional recommendation for the test, moderate quality of evidence).
DGP guidelines3:
Diagnosis:
- Low FeNO levels < 25 ppb (< 20 ppb in children) can be used to indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely.
- High FeNO levels > 50 ppb (> 35 ppb in children) can be used to indicate that eosinophilic inflammation and, in symptomatic patients, responsiveness to corticosteroids are likely.
Management:
- Patients with elevated FeNO levels are usually ICS-responsive. Elevated FeNO levels (especially FeNO levels > 50 ppb) during ICS therapy, despite clinical stability, argue against reducing the ICS dose.
- In children and adolescents, regularly monitored FeNO proved to be a meaningful parameter to predict asthma relapse after planned ICS discontinuation, even before the onset of clinical symptoms.
ATS guidelines4,5:
Diagnosis:
- Low FeNO levels < 25 ppb (< 20 ppb in children) can be used to indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely.
- Intermediate FeNO values between 25 ppb and 50 ppb (20 ppb and 35 ppb in children) should be interpreted cautiously and with reference to the clinical context.
- High FeNO levels > 50 ppb (> 35 ppb in children) can be used to indicate that eosinophilic inflammation and, in symptomatic patients, responsiveness to corticosteroids are likely.
Management:
- FeNO is beneficial and should be used in addition to usual care.
- Recommend the use of FeNO in monitoring airway inflammation in patients with asthma.
GINA guidelines7,8:
Diagnosis:
- High FeNO levels > 50 ppb in non-smokers are moderately associated with eosinophilic airway inflammation.
- FeNO levels above ≥ 20 ppb in adults with who have difficult-to-treat or severe asthma support the presence of type 2 airway inflammation.
Management:
- Measure FeNO as an adjunct to diagnostic evaluation in individuals with suspected asthma and to monitor airway inflammation.
FeNO testing with the NObreath®:
Regular FeNO measurements indicate levels of airway inflammation, which can help healthcare professionals personalise treatment plans for patients by helping titrate ICS dosing and evaluate patient adherence to treatment.
For over 48 years, Bedfont® Scientific Limited has specialised in designing and manufacturing breath analysis medical devices. Using innovative technology, we provide cutting-edge medical devices at affordable prices to improve accessibility and healthcare standards worldwide. Bedfont® manufactures the NObreath® FeNO device, a non-invasive breath testing device which can be used to measure airway inflammation for the diagnosis and management of asthma.
For more information on the NObreath® and FeNO testing, visit the NObreath® website.
References:
- Asthma pathway (BTS, NICE, SIGN) [Internet]. National Institute for Health and Care Excellence. 2024. [Cited Friday 17th January 2025]. Available from: https://www.nice.org.uk/guidance/ng244
- Louis R, Satia I, Ojanguren I, Schleich F, Bonini M, Tonia T, Rigau D, Ten Brinke A, Buhl R, Loukides S, Kocks JW. European Respiratory Society guidelines for the diagnosis of asthma in adults. European Respiratory Journal. 2022 Sep 1;60(3). DOI: 10.1183/13993003.01585-202.
- Lommatzsch M, Criée CP, de Jong CC, Gappa M, Geßner C, Gerstlauer M, Hämäläinen N, Haidl P, Hamelmann E, Horak F, Idzko M. Diagnosis and treatment of asthma: a guideline for respiratory specialists 2023-published by the German Respiratory Society (DGP) e. V. Pneumologie (Stuttgart, Germany). 2023 Jul 5. DOI: 10.1055/a-2070-2135.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR, American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American journal of respiratory and critical care medicine. 2011 Sep 1;184(5):602-15. PMCID: PMC4408724 PMID: 21885636.
- Khatri SB, Iaccarino JM, Barochia A, Soghier I, Akuthota P, Brady A, Covar RA, Debley JS, Diamant Z, Fitzpatrick AM, Kaminsky DA. Use of fractional exhaled nitric oxide to guide the treatment of asthma: an official American Thoracic Society clinical practice guideline. American journal of respiratory and critical care medicine. 2021 Nov 15;204(10):e97-109. PMCID: PMC8759314 PMID: 34779751.
- Zitt M, Oppenheimer J, Bernstein D, Boggs P, Dinakar C, Jain N, Katial N, Sands M, Szefler S. AAAAI/ACAAI joint statement of support of the ATS clinical practice guideline: interpretation of exhaled nitric oxide for clinical applications. 2012 [Internet]. 2014.
- Global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma. 2024. [Cited Friday 17th January 2025]. Available from: https://ginasthma.org/2024-report/
- Murugesan N, Saxena D, Dileep A, Adrish M, Hanania NA. Update on the role of FeNO in asthma management. Diagnostics. 2023 Apr 15;13(8):1428. DOI: 10.3390/diagnostics13081428.
- NHS England [Internet]. Quality and outcomes framework guidance for 24/25. 2024. [Cited 22nd April 2025]. Available from https://www.england.nhs.uk/publication/quality-and-outcomes-framework-guidance-for-2024-25/
- Healthline [Internet]. What you need to know abut FeNO testing for asthma. 2024. [Cited 22nd April 2025]. Available from: https://www.healthline.com/health/asthma/feno-test-asthma?c=1285499318383#takeaway

